
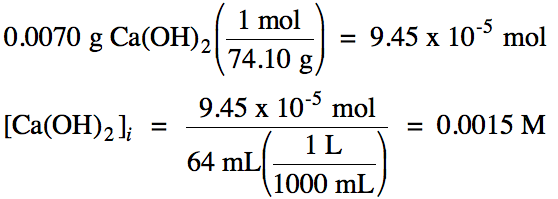
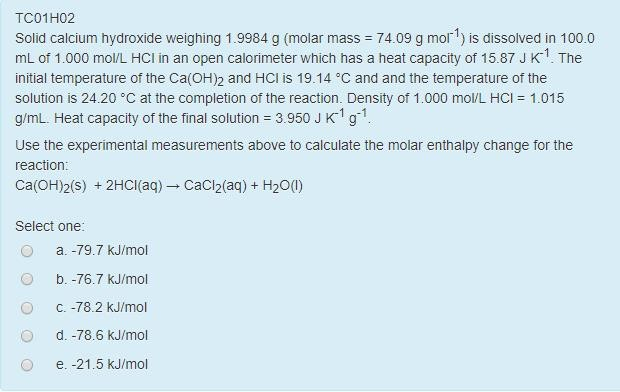
This means that, when 2.14 x 10¯ 4 mole per liter of CaF 2 dissolves, it produces 2.14 x 10¯ 4 mole per liter of Ca 2+ and it produces 4.28 x 10¯ 4 mole per liter of F¯ in solution. K sp = (5.71 x 10¯ 7) (5.71 x 10¯ 7) = 3.26 x 10¯ 13Įxample #2: Determine the K sp of calcium fluoride (CaF 2), given that its molar solubility is 2.14 x 10¯ 4 moles per liter.ġ) When CaF 2 dissolves, it dissociates like this:ģ) There is a 1:1 molar ratio between CaF 2 and Ca 2+, BUT there is a 1:2 molar ratio between CaF 2 and F¯. This means that, when 5.71 x 10¯ 7 mole per liter of AgBr dissolves, it produces 5.71 x 10¯ 7 mole per liter of Ag + and 5.71 x 10¯ 7 mole per liter of Br¯ in solution.Ĥ) Putting the values into the K sp expression, we obtain: In like manner, there is a 1:1 molar ratio between dissolved AgBr and Br¯ in solution. Given this value, how does one go about calculating the K sp of the substance? Here is a skeleton outline of the process:ġ) Write the chemical equation for the substance dissolving and dissociating.ģ) Insert the concentration of each ion and multiply out.Įxample #1: Determine the K sp of silver bromide, given that its molar solubility is 5.71 x 10¯ 7 moles per liter.ġ) When AgBr dissolves, it dissociates like this:ģ) There is a 1:1 molar ratio between the AgBr that dissolves and Ag + that is in solution. In the case of AgBr, the value is 5.71 x 10¯ 7 moles per liter. For very soluble substances (like sodium nitrate, NaNO 3), this value can be quite high, exceeding 10.0 moles per liter of solution in some cases.įor insoluble substances like silver bromide (AgBr), the molar solubility can be quite small. The molar solubility of a substance is the number of moles that dissolve per liter of solution.
#MOLAR MASS OF AG HYDROXIDE FULL#
Symbols, abbreviations, or full names for units of length,Īrea, mass, pressure, and other types.ChemTeam: Calculating the Ksp from Molar SolubilityĬalculating the K sp from the Molar Solubility You can find metric conversion tables for SI units, as wellĪs English units, currency, and other data. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.Ĭonversion calculator for all types of measurement units. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance.
#MOLAR MASS OF AG HYDROXIDE HOW TO#
This site explains how to find molar mass.įinding molar mass starts with units of grams per mole (g/mol). The reason is that the molar mass of the substance affects the conversion. To complete this calculation, you have to know what substance you are trying to convert. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.Ī common request on this site is to convert grams to moles. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. These relative weights computed from the chemical equation are sometimes called equation weights. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.įormula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.

This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Grams Aluminium Hydroxide to moles, or enter other units to convert below: Enter two units to convert From: You can do the reverse unit conversion from


 0 kommentar(er)
0 kommentar(er)
